Scientists from Evonik and the University of Reading performed a study to structurally characterize Evonik’s methionyl-methionine to understand how it could be better applied in aquafeeds.
Amino acids are the essential building blocks of important biological structures in our bodies and can have different forms depending on their chemical structure. Each amino acid form is a reflection of the other, the same as your right and left hand, so would not align if placed over each other.
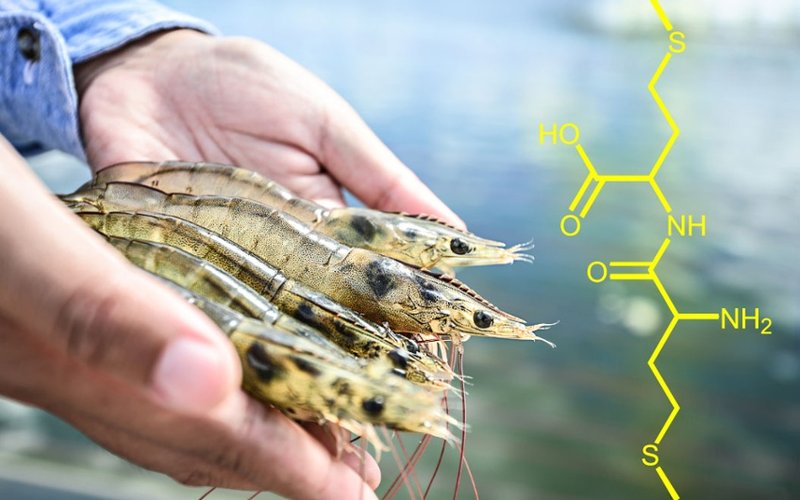
Methionine is one of these essential amino acids and is widely used in aquaculture to bulk up slow feeders, like shrimps, to promote better fish health and develop tastier shrimp. Unfortunately, if methionine is given as feed, shrimp gain little weight from it because most of it is either excreted or used for energy. It is also highly water soluble and can also disrupt ecosystems by increasing the growth of algae.
To solve this, Evonik developed methionyl-methionine, a supplement with original amino acid bonded to another methionine. This feed supplement contains the left-handed and right-handed form of methionine so it can be digested and used to build proteins, and is also more sustainable as it has lower water solubility. Methionyl-methionine contains two chiral centers, and so it can exist in four different forms: left-left, left-right, right-right and right-left.
This study used various techniques to look at crystals of the methionyl-methionine used in the Evonik feed supplement. One of the techniques was inelastic neutron scattering (INS) on the TOSCA spectrometer at the ISIS Neutron and Muon Source (ISIS) to build a fuller picture of the crystal’s structures. When the neutrons interact with the sample, scattering occurs and by measuring the energy difference between the incoming and outgoing neutrons, information about the crystal’s left-handed or right-handed form can be obtained at a small scale. Scientists were also able to use facilities in the Research Complex at Harwell, as well as STFC’s computing resources, to study the crystals. In combination with electron microscopy and other vibrational spectroscopy techniques, the scientists were able to study the differences between the methionyl-methionine forms. Both single-crystal and powder X-ray diffraction to determine two new methionyl-methionine crystal structures were also used.
Aside from differences in the shape and structure of the forms, researchers found that two of the forms showed very different solubilities which is important for knowing how to give this feed supplement in aquaculture. They also found no interaction between the two forms in a sample containing an equal mixture of them both at a small microscopic scale and a larger visible scale.
When the methionyl-methionine feed supplement is synthesized industrially, the different forms are likely to be in a mixture so insights like this could alter costs in the synthesis process. The insights from this study could lead to future research on the solubility differences between the forms and how this could be applied to other feeding techniques or feed supplements.
Read the study here.